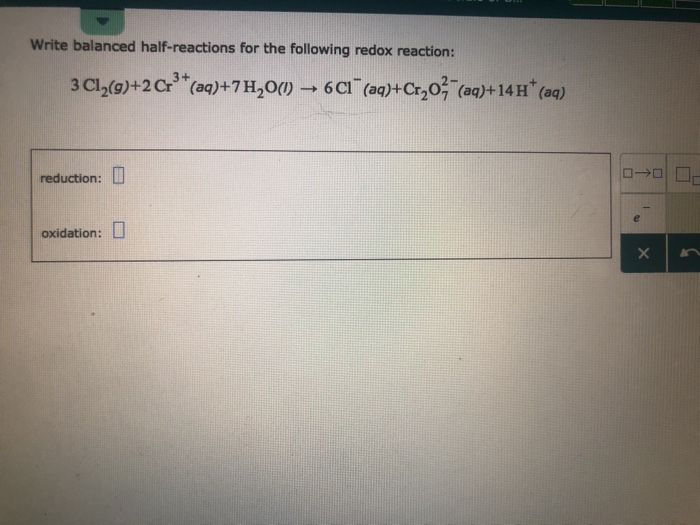
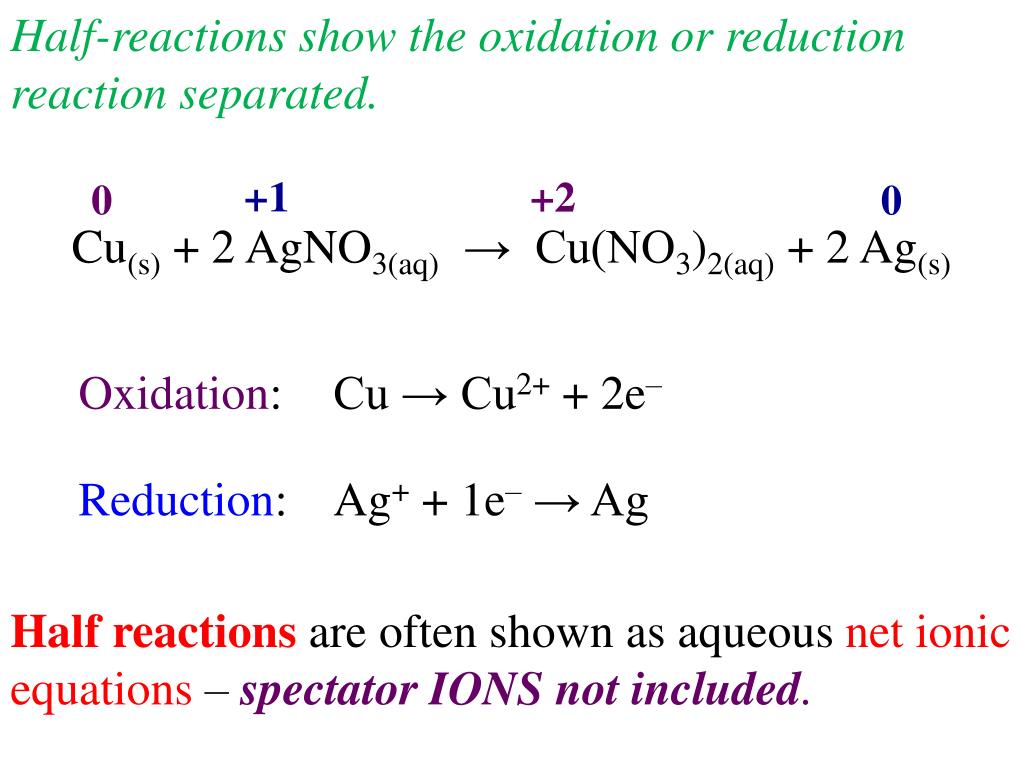
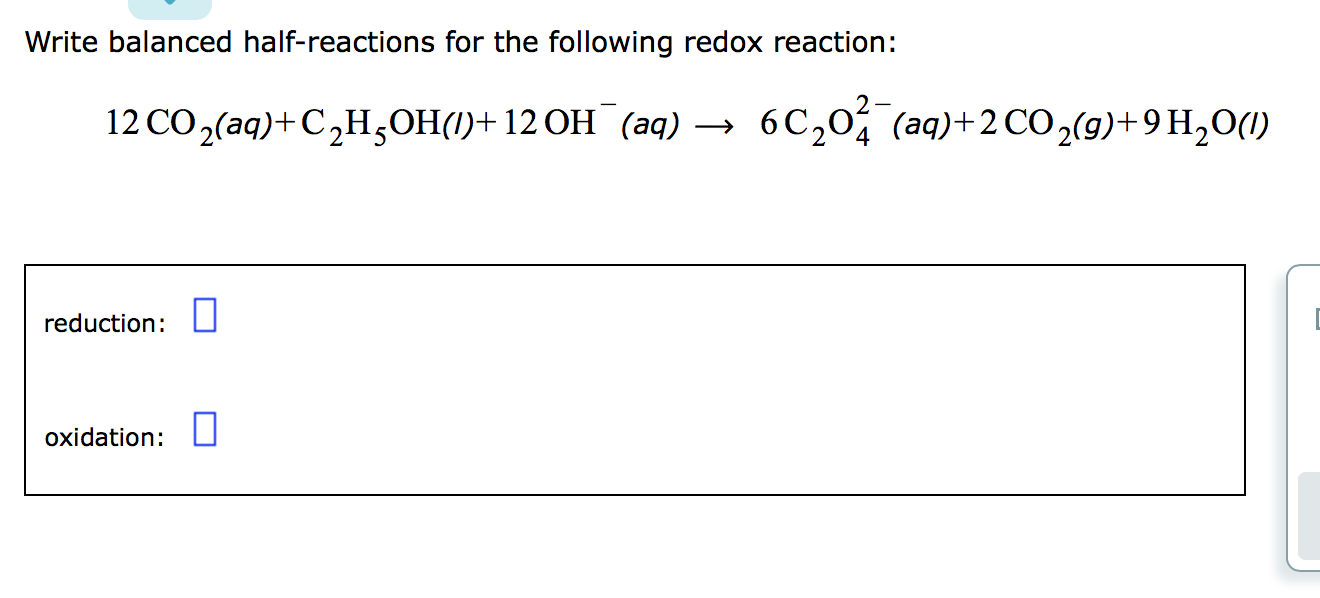
Awesome Tips About How To Write Redox Half Reactions
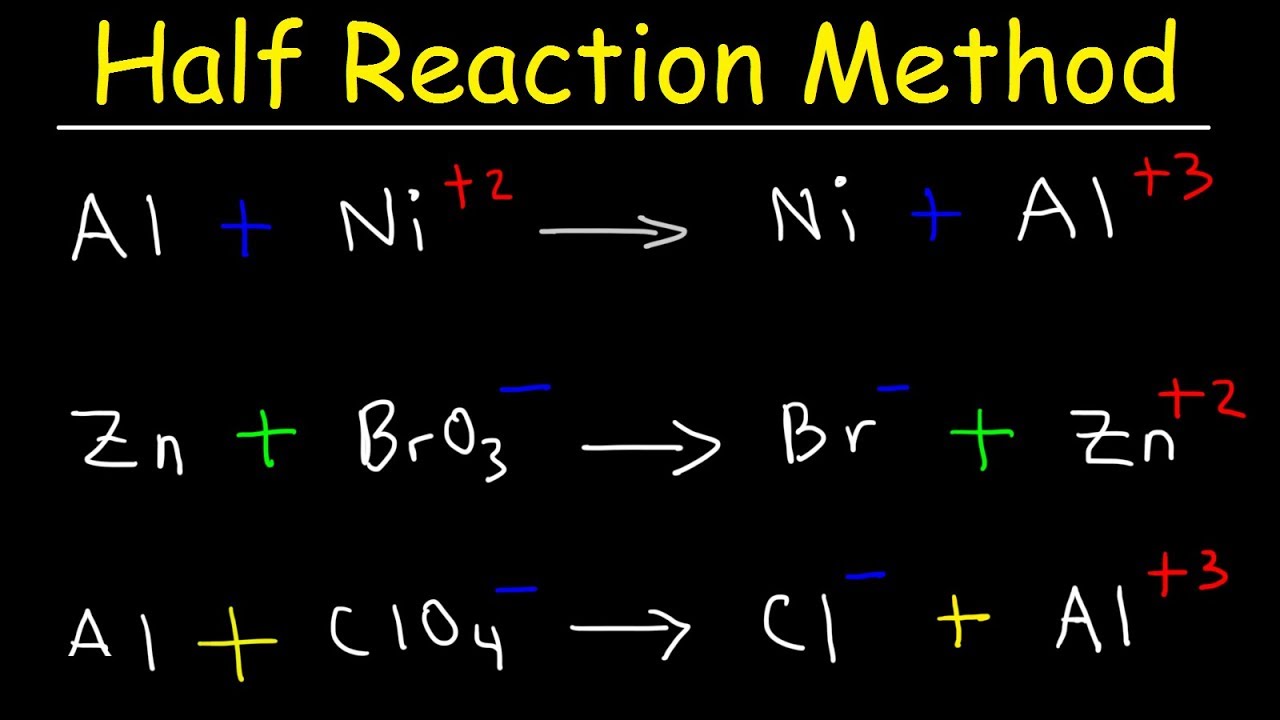
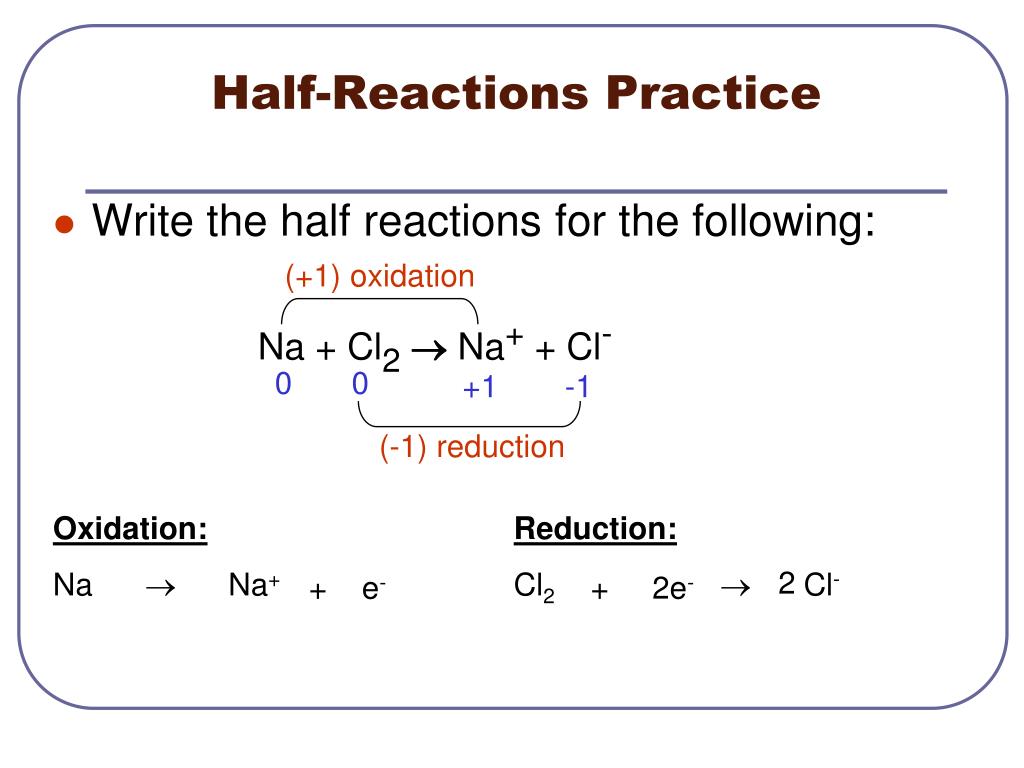
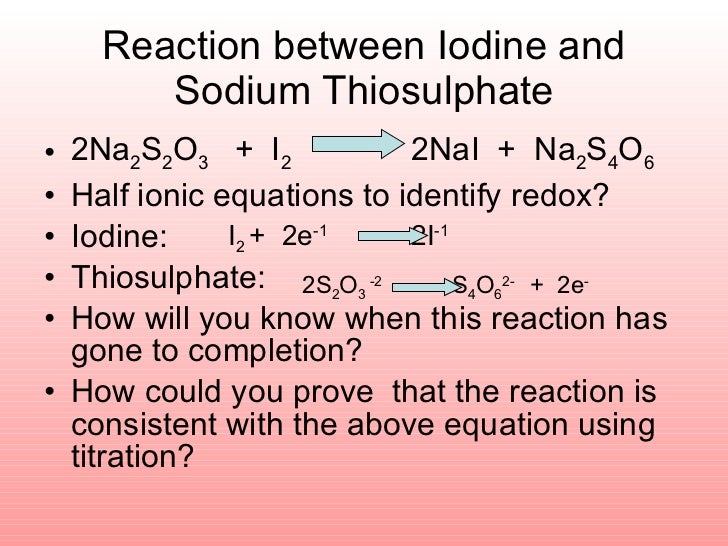
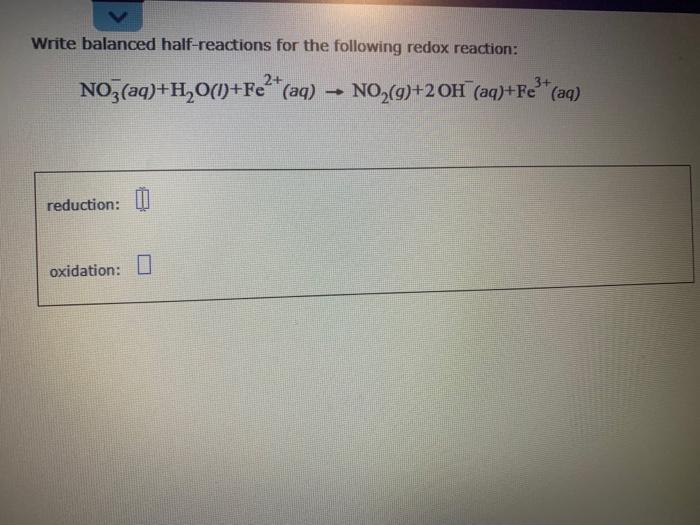
Redox reactions are often balanced by balancing each individual half reaction and then combining the two balanced half reactions.
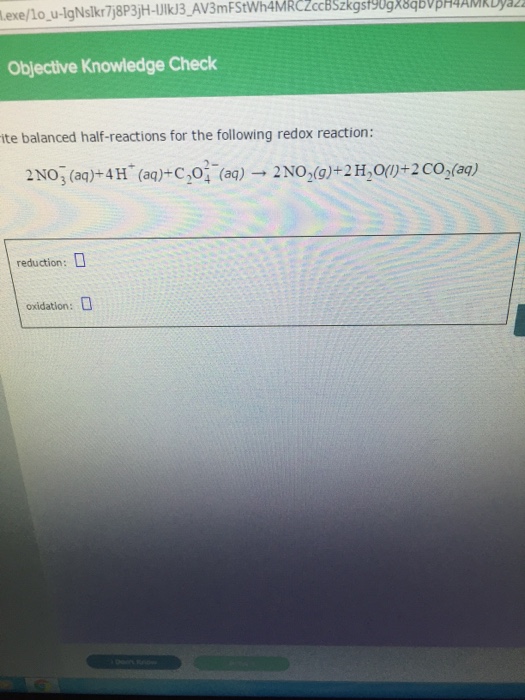
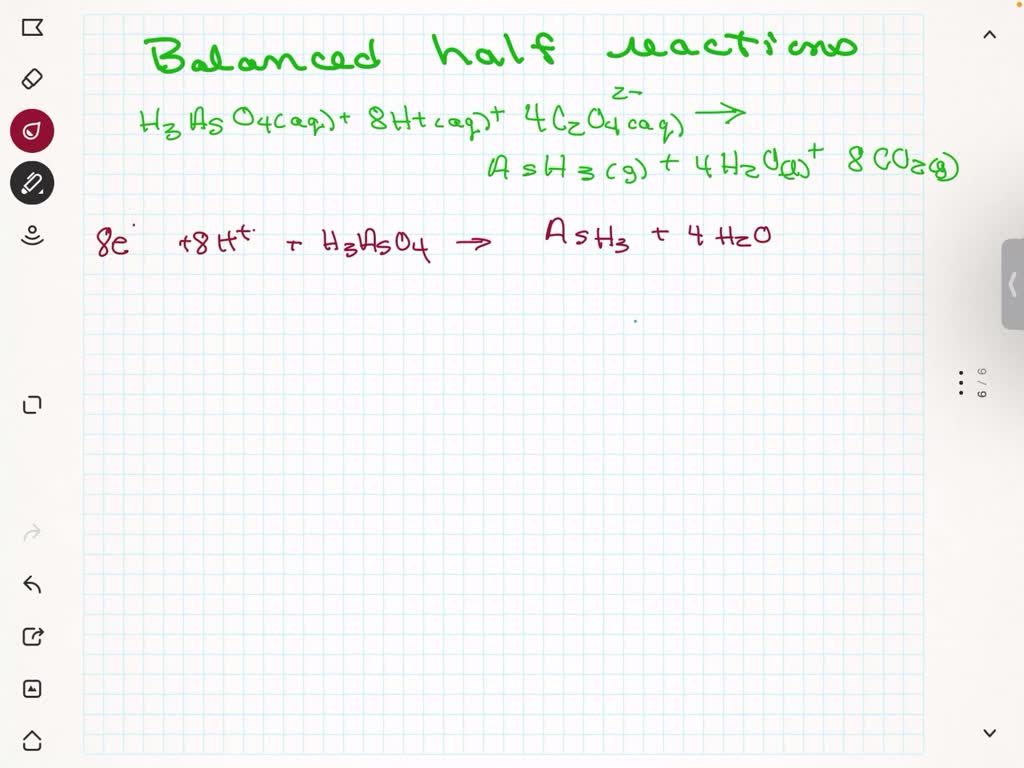
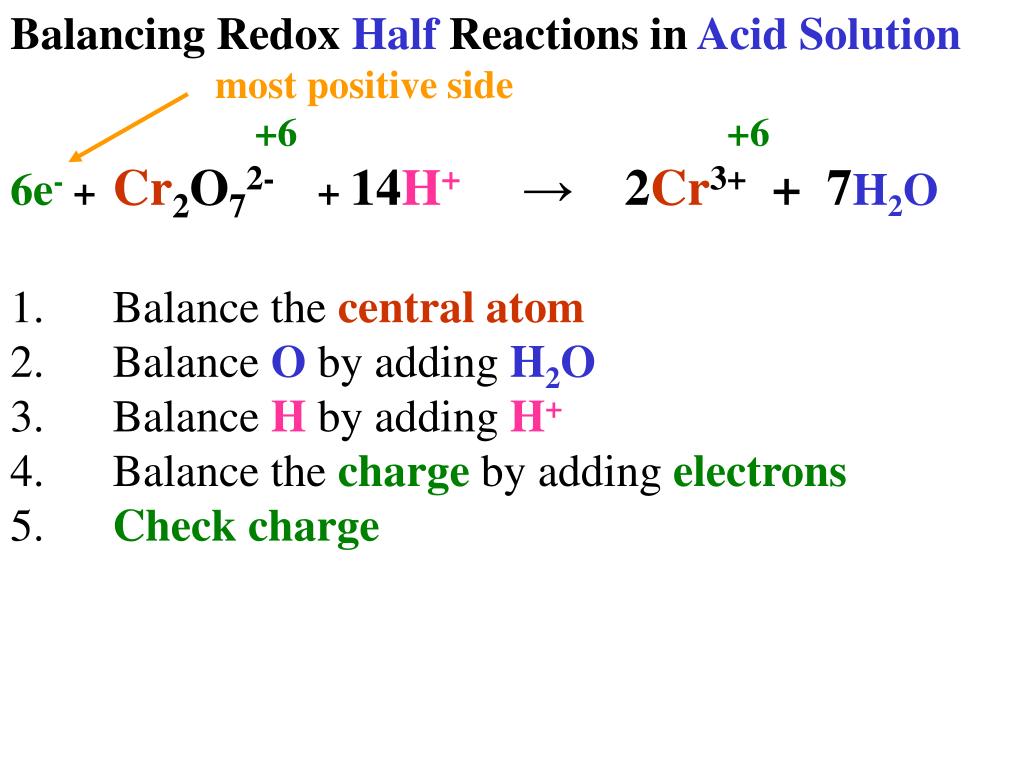
How to write redox half reactions. This technique involves breaking an equation into its two. Here are the 4 acid steps: There are three common methods to balance redox reactions:
Write the unbalanced redox reaction in its ionic form. 1.1m views 6 years ago new ap & general chemistry video playlist. 2) balance the oxygens (using h 2 o).
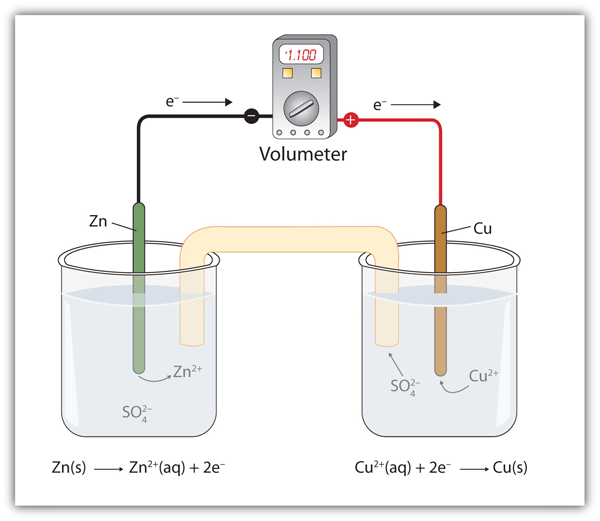
This chemistry video tutorial provides a basic introduction into the half. Redox reactions are all around us: A number assigned to an atom describing its degree of.
When balancing equations for redox reactions occurring in basic solution, it is often necessary to add oh⁻ ions or the oh⁻/h₂o pair to fully balance the equation. This lesson walks through how to write half reactions for oxidation and reduction given a particular. A type of chemical reaction where one or more electrons are lost.
3) balance the hydrogens (using h + ). A redox equation can be balanced using the following stepwise procedure: 1) balance the atom being reduced/oxidized.